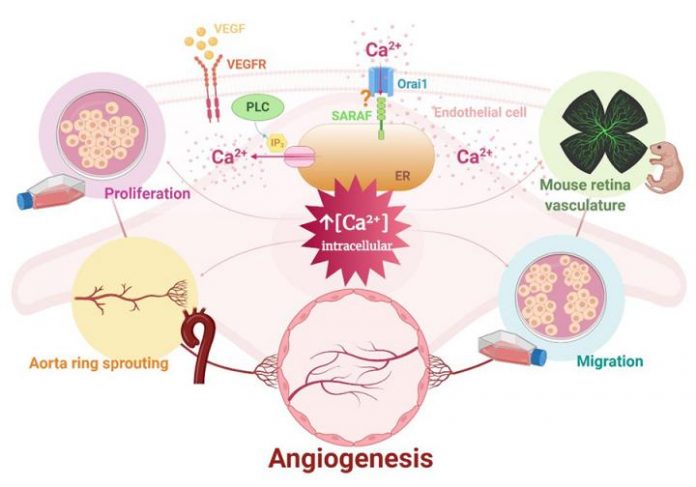
Angiogenesis is a process of new vessel formation that is activated both in physiological (tissue repair, reproduction, etc.) and pathological (myocardial infarction, diabetic retinopathy, cancer, etc.) conditions. The process is carried out by endothelial cells and includes their proliferation, migration and arrangement in tubes. Angiogenesis regulation is precise and is mainly mediated by pro-angiogenic factors, such as vascular endothelial growth factor (VEGF), which in turn promote different signalling pathways leading to an increase of intracellular Ca2+ concentrations.
The researchers from the Cardiovascular Pathophysiology group at the Institute of Biomedicine of Seville (IBiS) focused on precisely this point, demonstrating that the inhibition of certain proteins involved in the pathway’s regulation drastically affects the proper development of blood vessels. Specifically, these researchers demonstrated, for the first time, the involvement of SARAF, a SOCE regulatory protein, and Orai1, a subunit that forms the pore of the SOCE channel, in the VEGF-mediated activation of endothelial cells. Likewise, the research group has shown the importance of this Ca2+ pathway in the formation of new vessels and in the development of retinal vascularisation in neonatal mice. Thus, Orai1 and SARAF can be viewed as targets for the design of therapeutic strategies that could control angiogenesis in pathological situations such as cancer or retinopathies, or physiological situations such as post-infarction cardiac neovascularisation.