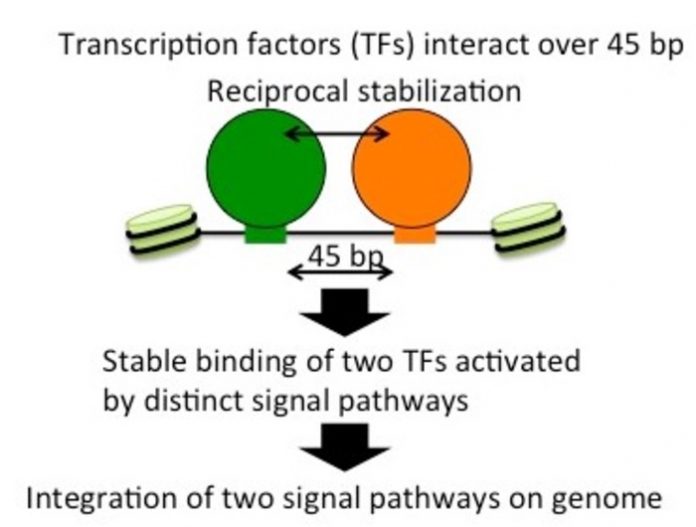
Scientists from Tokyo Metropolitan University have uncovered a unique mechanism where two transcription factors stabilize each other’s binding to DNA in fission yeast. They found that Atf1 and Rst2 help each other stably bind when they were close enough together. They both help transcribe a gene that deals with glucose poor environments but belong to entirely independent activation pathways. New insights like these can help scientists in the fight against cancer.
The popular picture of the DNA helix is of a long winding molecular thread, containing all the information required to create and sustain life. What is less known is how it is neatly packaged and stored inside cells: DNA is wound around protein structures known as histones, forming an elegant, tightly packed structure known as the chromatin. In order for molecular processes to actually use that information, the chromatin “opens,” making the DNA available for binding by transcription factors, proteins which help translate the DNA sequence made of base pairs (or “letters”) into messenger RNA (mRNA). This mRNA is then finally read by a ribosome to produce proteins based on the original blueprint.
How transcription factors (TF) bind to the chromatin is a key focus of biomedical research. Many cancers, for example, trace their origins to when this process goes wrong. A team led by Prof. Kouji Hirota of Tokyo Metropolitan University has been studying this process by looking at a simpler organism, the fission yeast, with a focus on how it responds to changes in their environment. Now, they have successfully caught a glimpse into the unique mechanism behind how transcription works in yeast cells responding to a lack of glucose in their surroundings.
When yeast cells are starved, it was known that transcription of the fbp1 gene was massively activated by two TFs, Atf1 and Rst2. The team investigated this process in depth and found not only that the activation of both was crucial to the function of fbp1, but that they actually helped stabilize each other. They were able to explicitly show that this was largely thanks to how close these sites were, usually a mere 45 base pairs apart. When extra lengths of DNA were introduced between the sites, the TFs suddenly could not help each other, and the chromatin closed, leaving both factors unbound. Their relative orientation along the twisting grooves of the helix also proved vital. Importantly, this effect was shown to be strong enough to counteract the effects of Tup11 and Tup12, co-repressors which help destabilize the random binding of independent TFs to the chromatin. All this suggests that this reciprocal relationship not only helps the TFs bind successfully, but also prevents either from attaching by themselves.
The curious thing is that these TFs are activated by completely independent chemical pathways. The process discovered by the team thus integrates these routes together into a signal “hub.” Though a single piece in a complex biochemical puzzle, this finding helps highlight an unappreciated mechanism by which different TFs interact and effectively integrate pathways together. The team hope this new insight can help in the fight against cancer and other related illnesses.