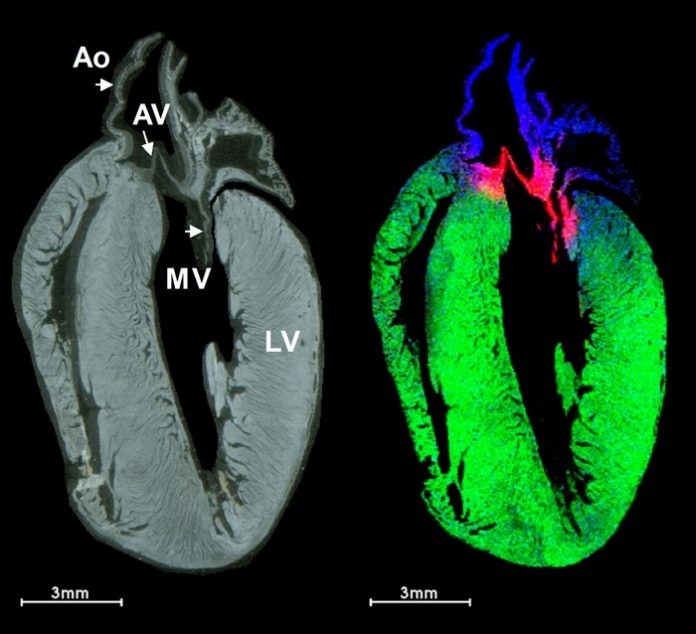
Myocardial infarction, or a heart attack, affects over 800,000 people in the U.S. every year. Following a heart attack, a scar forms on the heart, leading to poorer heart function, and ultimately, it could lead to heart failure. Current treatment options are limited and have wide-ranging side effects. Using a recently developed imaging technique, researchers at the Medical University of South Carolina, in collaboration with researchers from the University of Ottawa Heart Institute in Canada (BEaTS team, www.beatsresearch.com), unveiled new insights on how injectable collagen materials have therapeutic potential in the treatment of heart attacks. The team of MUSC researchers was led by Peggi Angel, Ph.D., associate professor in the College of Medicine. Their findings were published online in the Journal of the American Society for Mass Spectrometry on Oct. 29.
“Before, our collaborator could only detect the target of the therapy,” Angel explained, “whereas we can actually detect the therapeutic collagen peptide. We know where it has spread in the myocardial infarct. I think it is a better way of detecting the effectiveness of the treatment and should lead to new therapies, as we will know the exact molecule linked to the site of healing.”
In this study, Angel’s team used collagen hydrogels to study how introduced collagen affects and interacts with heart attack scars. Hydrogels are large groups of molecules consisting mostly of water. The high-water content makes them useful in therapeutics because they can carry treatments and be accepted into the body. Using a new technique, they were able to distinguish between the heart’s scar collagen and the introduced biomaterials as well as analyze their distribution throughout the heart attack wound.
Collagen is a well-known protein often used by diet and skincare companies. There are 28 different collagen proteins found throughout the body and each has multiple functions, depending on its structure and location. Angel and colleagues studied fibrillar collagens, which are different than those found in products at the store, in wound healing in the heart.
“It’s a very specific sequence compared with what may be found on the label of a commercially available collagen supplement,” explained Angel.
Using an injectable collagen material, the BEaTS team developed a therapeutic material that localizes to the heart attack area. “The biomaterial prepared by the BEaTS team keeps the therapy in a certain location, such as the scar,” explained Angel. “The cells can sense the biomaterials presence and change how they respond.”
To see where the introduced therapeutic collagen went, Angel used MALDI-IMS, matrix-assisted laser desorption/ionization-imaging mass spectrometry. Mass spectrometry is a relatively new technique that can detect charged molecules from tissue samples.
“Mass spectrometry detects ions, a molecule that has a positive or negative charge, like a little magnet. Mass spectrometry works by guiding that little magnet to a detector, and we can use different ways to direct them to detect different types of molecules,” said Angel.
One method of detection is IMS, or imaging mass spectrometry. In MALDI-IMS, researchers can pinpoint ions to a particular location in the tissue, in this case the heart. This allows researchers to gather spatial information about potential treatments, which is valuable for developing therapeutics.
In this study, laboratory mice underwent an experimental heart attack. The mice were then treated with human recombinant collagen hydrogels injected directly into the heart muscle and monitored by MALDI-IMS. To identify the injected collagens, rather than the collagens made naturally within the mouse, the researchers injected human collagen. Because collagen proteins are unique to each species, the different collagens can be detected by a mass spectrometer.
The work by Angel and her team offers a new technique for studying biomaterial injections. Previous studies were only capable of detecting the end result of a particular treatment, but this study allows for visualization of the treatment and it’s spread throughout the heart and wound area. By analyzing how treatments and therapeutics are distributed throughout a wound, researchers can evaluate the effectiveness of therapies and hopefully develop new, cleaner techniques to avoid side effects.
In the future, IMS might be used to target where therapies are most effective and determine proper delivery location and timing. Previous techniques generally required researchers to know and label what they would be looking for beforehand. Mass spectrometry does not require prior knowledge or information to do experiments and thus allows for novel discoveries.
“You generally need some type of tag or known molecule to target. Mass spectrometry is easily used as a discovery technique. We can detect all sorts of molecules ranging from metabolites to lipids to proteins and even up to DNA,” said Angel.
This, combined with a greater understanding of how collagen dynamics can affect heart function, helps researchers to develop new therapies that lead to a more functional heart following a heart attack. It is Angel’s goal, she said, to continue to develop innovative ways to visualize and treat disease.